The inverse electron-demand Diels–Alder reaction as a new methodology for the synthesis of 225Ac-labelled radioimmunoconjugates†
Abstract
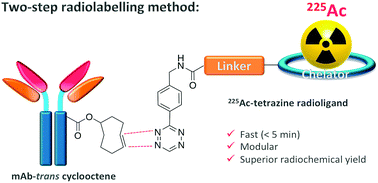
The inverse electron-demand Diels–Alder reaction between tetrazine (Tz) and trans-cyclooctene (TCO) facilitates the efficient radiosynthesis of 225Ac-labelled radioimmunoconjugates in a two-step method, outperforming conventional approaches based on isothiocyanate couplings.


 Please wait while we load your content...
Please wait while we load your content...