Direct access to α-sulfenylated amides/esters via sequential oxidative sulfenylation and C–C bond cleavage of 3-oxobutyric amides/esters†
Abstract
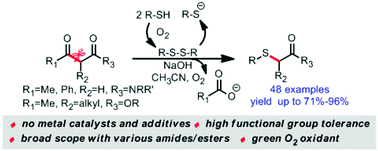
An efficient, environmentally benign and unprecedented synthesis of various α-sulfenylated amides/esters has been developed under oxygen atmosphere. The reaction shows good functional group tolerance and excellent chemo/regioselectivity. All the desired products were obtained in moderate to excellent yields, even on the gram scale. Practically, the related α-thiol Weinreb amide can be readily transferred to a series of prospective compounds, and selenium atom can be introduced to the α-sites of the amides in high yields.


 Please wait while we load your content...
Please wait while we load your content...