Nine-step total synthesis of (−)-strychnofoline†
Abstract
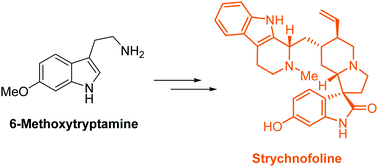
Strychnofoline is a Strychnos alkaloid that has unique spirooxindole architecture and possesses important anticancer activity. Here, we have, for the first time, reported the enantioselective synthesis of strychnofoline proceeding in only nine steps from commercially available 6-methoxytryptamine. The efficiency of the synthesis derives from the use of two sequential transformation steps in the catalytic asymmetric construction of the spiro[pyrrolidine-3,3′-oxindole] motif in a facile manner. Our route is amenable to the synthesis of other natural and synthetic analogs of bioactive spirooxindole alkaloids to access their therapeutic potential.
- This article is part of the themed collection: Natural product synthesis


 Please wait while we load your content...
Please wait while we load your content...