Regioselective direct arylation of indoles on the benzenoid moiety
Abstract
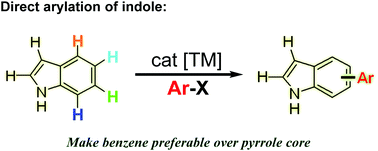
Recent advances in transition metal-catalyzed selective C–H functionalization of indoles have garnered tremendous attention. Great efforts have been devoted to C2 and C3 arylation because of the inherent reactivity of the pyrrole ring. Until recently, elegant methods have been developed to enable selective direct arylation on the benzenoid moiety at C4, C5, C6, and C7. This review highlights the contributions made in benzenoid direct arylation of indoles and presents their potential in organic synthesis.


 Please wait while we load your content...
Please wait while we load your content...