In situ electrochemical development of copper oxide nanocatalysts within a TCNQ nanowire array: a highly conductive electrocatalyst for the oxygen evolution reaction†
Abstract
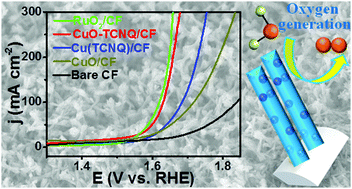
It is highly desired to develop efficient earth-abundant electrocatalysts for the oxygen evolution reaction (OER) in alkaline media. In this communication, we report the in situ electrochemical conversion of a nanoarray of Cu(tetracyanoquinodimethane), Cu(TCNQ), an inorganic–organic hybrid, on Cu foam into CuO nanocrystals confined in a highly conductive nanoarray via anode oxidation. As a 3D catalyst electrode, the resulting CuO–TCNQ/CF shows high OER activity and demands an overpotential of only 317 mV to drive a geometrical catalytic current density of 25 mA cm−2. Notably, this catalyst also demonstrates strong long-term electrochemical durability. This study provides us with a universal strategy toward topotactic room-temperature preparation of conductive nanoarrays with confined transition metal nanocatalysts for practical applications.


 Please wait while we load your content...
Please wait while we load your content...