Pd/Cu dual catalysis: highly enantioselective access to α-substituted α-amino acids and α-amino amides†
Abstract
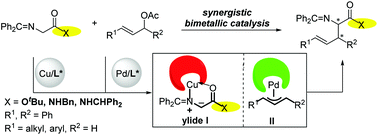
The asymmetric allylation of glycine iminoesters has been accomplished through a synergistic Pd/Cu catalyst system, affording a range of α-substituted α-amino acids in high yields and with excellent enantioselectivities (88 → 99% ee). The introduction of a Cu-P,N-metallocenyl complex-activated glycine iminoester to the chiral palladium-catalyzed allylic allylation process is crucial owing to its high reactivity and excellent enantioselectivities. Importantly, this Pd/Cu dual catalysis strategy can be used for the asymmetric allylic alkylation of prochiral glycine amide derivatives, which could be further utilized to synthesize biologically important vicinal diamines.


 Please wait while we load your content...
Please wait while we load your content...