A tethering directing group strategy for ruthenium-catalyzed intramolecular alkene hydroarylation†
Abstract
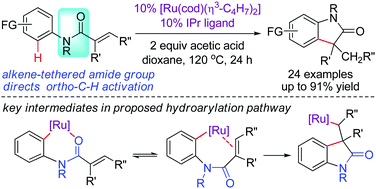
We report a new catalyst design for N-heterocycle synthesis that utilizes an alkene-tethered amide moiety as a directing group for aromatic C–H activation. This tethering directing group strategy is demonstrated in a ruthenium-catalyzed intramolecular alkene hydroarylation with N-aryl acrylamides to form oxindole products.


 Please wait while we load your content...
Please wait while we load your content...