Stereoselective synthesis of sulfonated 1-indenones via radical-triggered multi-component cyclization of β-alkynyl propenones†
Abstract
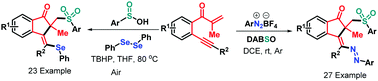
New radical-triggered multi-component cyclizations of β-alkynyl propenones have been developed, leading to 50 examples of sulfonated 1-indenones with generally good yields and high levels of stereoselectivity. The oxidant-free azosulfonylation of β-alkynyl propenones with aryldiazonium salts and DABSO was realized under the neutral–redox conditions where TBHP enabled the direct selenosulfonylation of β-alkynyl propenones by combining sulfinic acids and diphenyl diselenide. This protocol features a broad substrate scope, high functional group tolerance and mild reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...