An oxidative cross-coupling reaction of 4-hydroxydithiocoumarin and amines/thiols using a combination of I2 and TBHP: access to lead molecules for biomedical applications†
Abstract
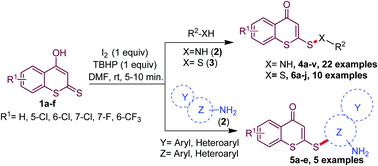
A metal-free I2/TBHP induced highly atom economic and operationally simple oxidative cross-coupling reaction has been developed for the direct synthesis of sulfenamides/sulfanes/disulfides from the reaction of 4-hydroxydithiocoumarin and amines/thiols. The novelties of the present protocol are unprecedented S–C bond formation in addition to S–N and S–S bonds, shorter reaction time, mild and environmentally benign reaction conditions, functional group tolerance and moderate to excellent yields. Moreover, the four newly synthesized compounds namely 4q, 6d, 6e and 7a exhibit anti-proliferative activity against the breast cancer cell line MCF7, and may be lead molecules for future drug development.


 Please wait while we load your content...
Please wait while we load your content...