A nickel-catalyzed anti-carbometallative cyclization of alkyne–azides with organoboronic acids: synthesis of 2,3-diarylquinolines†
Abstract
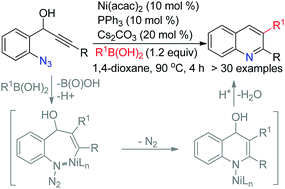
An anti-carbonickelative cyclization via reversible alkenylnickel E/Z isomerization of 2-azido phenyl propargyl alcohols with aryl boronic acids is achieved using Ni(acac)2 as the catalyst to access 2,3-diaryl quinolines. It represents a rare example of trapping the vinyl metal intermediate with a nitrogen center, a non-carbon center electrophile.
- This article is part of the themed collection: 20th Anniversary of the CRSI: Celebrating Indian Chemistry in ChemComm


 Please wait while we load your content...
Please wait while we load your content...