New approaches to the lithiation kinetics in reaction-limited battery electrodes through electrochemical impedance spectroscopy
Abstract
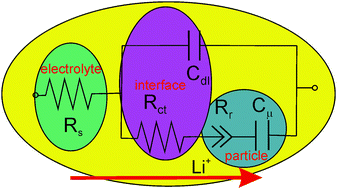
Electrochemical impedance spectroscopy is a widely employed technique probing kinetic limitations in the charging of battery electrodes. Hindrance mechanisms locate at the interfaces between the active material and the electrolyte, and in the bulk of the reacting compound. Rate-limiting mechanisms are viewed as resistive circuit elements and can be extracted using standard impedance analyzers. Classical impedance models consider charge transport, mainly ion diffusion as slower carrier, as the principal kinetic limitation impeding full electrode charging. This is indeed the case for many technologically relevant battery compounds. In other instances, instead of being diffusion-limited, electrodes may undergo charging limitation caused by the kinetics of the reduction reaction itself. Specific impedance models for reaction-limited mechanisms are summarized here and proved for relevant electrode compounds, in particular for conversion or alloying electrodes in which Li+ intake produces a full rearrangement of the lattice structure with significant atomic displacement.


 Please wait while we load your content...
Please wait while we load your content...