Direct observation of Ru-alkylidene forming into ethylene in ring-closing metathesis from hyperpolarized 1H NMR†
Abstract
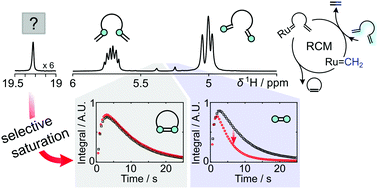
Ring-closing metathesis was monitored using real-time NMR of 1H hyperpolarized olefins at room temperature. By applying a selective saturation to an observable intermediate, its protons were found to transfer to ethylene. The intermediate was thus identified as a Ru-alkylidene species, which appears in the ethylene formation pathway.


 Please wait while we load your content...
Please wait while we load your content...
