The region-specific segregation and catalytic activity of gold–silver nanoparticles†
Abstract
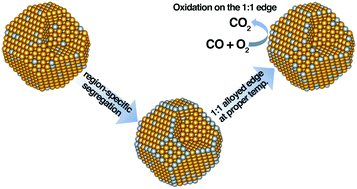
A coordination-dependent interatomic interaction is developed for Ag atoms and applied to simulate Au–Ag alloy nanoparticles ranging from 2.6 nm to 4.5 nm with different composition ratios at different temperatures. Using the lattice Monte Carlo method, we found that Ag segregates preferentially on the edges of nanoparticles. The region-specific segregation is revealed by our study and it indicates that depending on the size and temperature, Ag may segregate on the edges only, whereas its segregation on flat surfaces is negligible. Assuming that equimolar Au–Ag edges maximize the catalytic synergistic effect, we predicted the size and composition dependent optimal reaction temperatures for the Au–Ag catalyzed CO oxidation, which agree with the available experimental temperatures very well. Our study demonstrates that the region-specific segregation not only complements the details of surface segregation but is also very important for the utilization and tuning of faceted bimetallic nanoparticles.


 Please wait while we load your content...
Please wait while we load your content...