Ni(OH)2–Fe2P hybrid nanoarray for alkaline hydrogen evolution reaction with superior activity†
Abstract
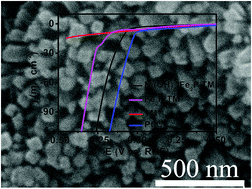
It is highly attractive to develop efficient hydrogen evolution reaction (HER) electrocatalysts under alkaline conditions. In this communication, we report the preparation of amorphous Ni(OH)2 decorated Fe2P nanoarray on Ti mesh (Ni(OH)2–Fe2P/TM) via electrodeposition. As a 3D electrode for the hydrogen evolution reaction, such Ni(OH)2–Fe2P/TM demonstrates superior catalytic activity. Moreover, the as-prepares electrocatalyst requires an overpotential of only 76 mV to drive a current density of 10 mA cm−2, which is 94 mV less than that for Fe2P/TM. It also shows strong long-term electrochemical durability with its catalytic activity being maintained for at least 20 h.


 Please wait while we load your content...
Please wait while we load your content...