Low-intensity focused ultrasound (LIFU)-activated nanodroplets as a theranostic agent for noninvasive cancer molecular imaging and drug delivery
Abstract
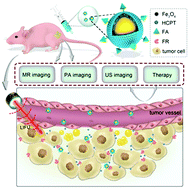
Theranostics is a new trend in the tumor research field, which involves the integration of diagnostic and therapeutic functions using imageable nanoparticles coupled with therapeutic drugs. Imaging-guided targeted delivery of therapeutics and diagnostics using nanocarriers hold great promise to minimize the side effects of conventional chemotherapy. Ultrasound microbubbles have been employed as theranostic agents over the last decade, which provide both real-time dynamic imaging for diagnosis and precise control for targeted tumor therapy. However, the intrinsic defects of microbubbles such as poor tissue penetration, short circulation time and instability hinder microbubble-based theranostic applications. In recent years, liquid-to-gas transitional perfluorocarbon nanoparticles have been developed as promising diagnostic and therapeutic nanoagents to solve the abovementioned problems. In this study, phase-changeable, folate-targeted perfluoropentane nanodroplets loaded with 10-hydroxycamptothecin (HCPT) and superparamagnetic Fe3O4 (denoted as FA-HCPT-Fe3O4-PFP NDs) are prepared and investigated for multimodal tumor imaging and targeted therapy. After intravenous administration into nude mice bearing SKOV3 ovarian cancer, FA-HCPT-Fe3O4-PFP NDs exhibit the ability to enhance MR and PA imaging. Furthermore, after the phase transition activated by low-intensity focused ultrasound (LIFU) sonication, FA-HCPT-Fe3O4-PFP NDs remarkably enhance US imaging at the tumor location. Meanwhile, the HCPT released from FA-HCPT-Fe3O4-PFP NDs during the liquid-to-gas transition provides a therapeutic effect on tumor cells with relatively low side effects to normal tissue. Therefore, the combination of LIFU and FA-HCPT-Fe3O4-PFPNDs presents an ideal modality for tumor-targeted theranostics.


 Please wait while we load your content...
Please wait while we load your content...