Polytyrosine nanoparticles enable ultra-high loading of doxorubicin and rapid enzyme-responsive drug release†
Abstract
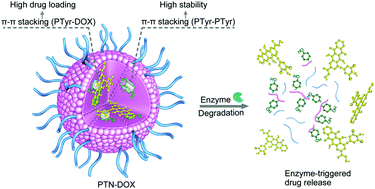
Despite the great significance of clinically viable nanovehicles, very few of them exhibit stability and high anticancer drug loading with fast intracellular drug release. Herein, we report that polytyrosine nanoparticles (PTNs) self-assembled from poly(ethylene glycol)-b-poly(L-tyrosine) block copolymer enable the ultra-high loading and rapid enzyme-responsive release of doxorubicin (DOX). Notably, PTNs achieve a remarkably high DOX loading of 63.1 wt% likely due to the existence of strong π–π stacking between polytyrosine and DOX, as shown by UV-vis analysis. Additionally, PTNs present a high docetaxel loading of 17.5 wt%. Furthermore, PTNs exhibit good colloidal stability in 10% FBS, but are quickly de-stabilized by proteinase K. Interestingly, ca. 90% of DOX is released under 6 U mL−1 proteinase K in 24 h or in RAW 264.7 cells in 8 h. The DOX-loaded PTNs display efficient delivery and release of DOX in both RAW 264.7 cells and HCT-116 human colorectal cancer cells, achieving a better in vitro antiproliferative effect than the clinically used liposomal DOX formulation. Thus, these polytyrosine nanoparticles appear to be a potentially viable platform for the controlled delivery of anthraquinone anticancer agents.


 Please wait while we load your content...
Please wait while we load your content...