An approach combining enzymatic 18O-labeling and label-free methods for the quantitative dynamic analysis of hemogen phosphorylation
Abstract
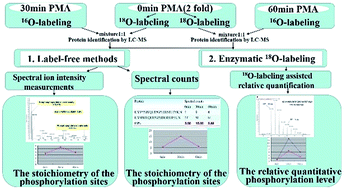
Phosphorylation is a key event in many biological processes, and the temporal and quantitative dynamics of protein phosphorylation is crucial to understand the signaling processes in cells. Stable isotope coding is believed to be a powerful technique for relative quantification of the modification level, whereas it is a challenge to obtain the phosphorylation stoichiometry at each phosphosite of the protein. Label-free methods of quantification directly using LC-MS can be introduced to achieve this goal just by comparing the ion peak intensity or spectra counting of a phosphorylated and its corresponding non-phosphorylated peptides eluting in the same run. In this study, we integrated enzymatic 18O-labeling and two kinds of label-free methods to explore the temporal phosphorylation changes over different stimulation times using phorbol myristate acetate (PMA) for hemogen, an important protein in hematopoietic development and neoplasms. Complementary methods lead to the concurrent identification of the relative quantification and the stoichiometry of site-specific phosphorylation. What's more, LC-MS/MS combined with no enzyme specificity searching parameters was applied to assign novel phosphosites.


 Please wait while we load your content...
Please wait while we load your content...