Investigation of ethosuximide stability under certain ICH-recommended stress conditions using a validated stability-indicating HPLC method
Abstract
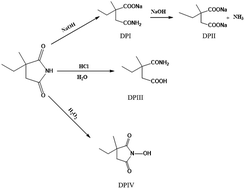
A study of the chemical stability of the antiepileptic drug ethosuximide (ESX) under certain ICH-recommended stress conditions was conducted for the first time. A stability-indicating HPLC method was applied using a mobile phase containing 0.05 M sodium dihydrogen phosphate and methanol (90 : 10 v/v) at pH 3.5 and a Promosil C18 column for separation of ESX from its potential degradation products with UV detection at 210 nm. The method validity was confirmed showing a linearity range of 2.0–30.0 μg mL−1 with a lower limit of detection of 0.14 μg mL−1. The results of the developed method showed good agreement with those obtained by the USP official method. The stability of ESX was investigated by subjecting it to some stress conditions of the ICH guideline such as acidic, alkaline and oxidative conditions. The drug was liable to degradation under these conditions yielding specific degradation products. The validated stability-indicating HPLC method efficiently separated the drug from all of the formed degradation products proving its suitability for purity and stability testing.


 Please wait while we load your content...
Please wait while we load your content...