Modeling the response of a control-released ion-selective electrode and employing it for the study of permanganate oxidation kinetics†
Abstract
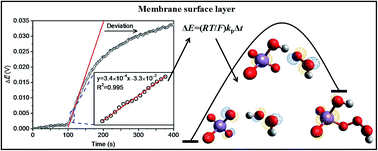
Although polymeric membrane ion-selective electrodes (ISEs) based on outward ion fluxes have been found analytically useful, there is still a lack of a theoretical framework for this detection system. In this study, we attempted to model the response of this kind of permanganate ISE and employed this ISE to analyze the rapid MnO4−/H2O2 reaction. This response is attributed to H2O2 oxidation with MnO4− that is released from the inner solution to the membrane surface layer. The results show that the experimental data can be fitted well to the proposed model that is elucidated mathematically from the viewpoint of chemical kinetics. The second-order rate constant is determined at a near neutral pH and is in agreement with the acid dissociation law to provide the specific value of 370 M−1 s−1. The kinetic mechanism was then investigated by performing DFT calculations. Via analysis of the Mn–O bond length and the HOMO orbital, it has been found that the studied redox system functions similarly as the so-called hydrogen abstraction mechanism with an energy barrier of 24.5 kcal mol−1. This study is considered to be the first report on the simulation of MnO4− attack at the O–H bond. On the basis of the transition state theory and previous studies on MnO4− attack at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–H bonds, the relationship between the experimental rate constant and computational energy barrier is finally constructed. The result indicates the validity of our proposed method and makes the control-released ISE a very promising platform to study the kinetics.
C and C–H bonds, the relationship between the experimental rate constant and computational energy barrier is finally constructed. The result indicates the validity of our proposed method and makes the control-released ISE a very promising platform to study the kinetics.


 Please wait while we load your content...
Please wait while we load your content...