A liquid chromatographic method for separation of sacubitril–valsartan and their stereoisomeric impurities
Abstract
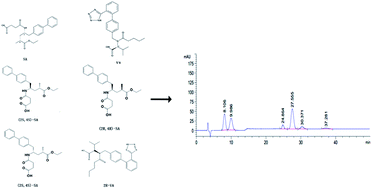
Sacubitril–valsartan (SV) is a first-in-class inhibitor of the angiotensin II receptor and neprilysin for the treatment of chronic heart failure (HF) and hypertension. A stereoselective normal-phase high performance liquid chromatographic method was developed and validated for separation of sacubitril (SA), valsartan (VA) and their stereoisomeric impurities. Chromatography was performed using mobile phase A of n-hexane with 0.1% TFA added and mobile phase B comprising ethanol, isopropanol and TFA (80 : 20 : 0.1, v/v/v) and delivered at a flow rate of 1.0 mL min−1 on a Chiralcel OJ-H column (250 mm × 4.6 mm, 5 μm). The stereoisomers were monitored at a wavelength of 254 nm and the whole separation was achieved within 50 min. The method was validated in terms of specificity, linearity (R2 ≥ 0.998), accuracy (98.3–99.5%), precision (%RSD ≤ 1.82), limit of detection (0.06 μg mL−1 and 0.10 μg mL−1) and limit of quantification (0.2 μg mL−1 and 0.3 μg mL−1). The sample solution and mobile phase were found to be stable for at least 48 hours. The final optimized method was successfully applied to separate sacubitril–valsartan from their stereoisomers and showed characteristics of good repeatability and accuracy for quantitative determination of the stereoisomers in bulk drug.


 Please wait while we load your content...
Please wait while we load your content...