Assessment of the performance of triphenylphosphine for the voltammetric determination of elemental sulphur in cosmetic products
Abstract
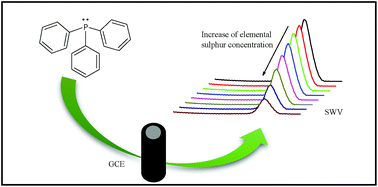
In this paper, an alternative voltammetric method for the determination of elemental sulphur in cosmetic products is presented. It is based on the decrease of triphenylphosphine oxidation current in the presence of elemental sulphur by using a glassy carbon electrode. A solution of 2% (m/v) acetic acid and 0.6 mol L−1 sodium acetate in methanol was used as a supporting electrolyte. The experimental conditions for indirect determination of elemental sulphur were established. Using square-wave voltammetry, the analytical curve was linear in the elemental sulphur concentration range of 9.94–271 μmol L−1, with a detection limit of 2.59 μmol L−1. The method was successfully applied to determine elemental sulphur in soap bars and anti-acne cream, without any preliminary sample treatment, therefore, it is shortened and simplified. The results obtained with the indirect voltammetric method were not statistically different in comparison with a titrimetric one, at a 95% confidence level. Additionally, excellent recovery percentages were obtained, proving no matrix interferences.


 Please wait while we load your content...
Please wait while we load your content...