Spectroelectrochemical study of the AMP-Ag+ and ATP-Ag+ complexes using silver mesh electrodes†
Abstract
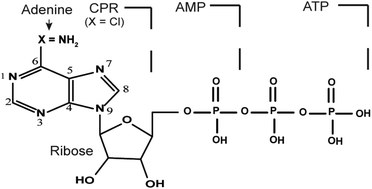
In this study, electrochemical reaction mechanism of adenosine monophosphate (AMP) and adenosine triphosphate (ATP) on a silver mesh was investigated in acetate buffer using spectroelectrochemical technique. The results indicate that AMP (or ATP) can form a complex with silver ion originating from a silver mesh when a positive potential was applied. In these complexes, silver ion coordinates with AMP or ATP via their phosphate group. However, when a negative potential was applied, the formed complex disappeared. The complex reaction is therefore an electrochemically reversible process. Further studies using surface-enhancement Raman spectroscopy (SERS) have shown that AMP (or ATP) has a parallel or perpendicular orientation to the silver mesh surface, which is governed by their different binding sites (adenine ring, ribose, and phosphate groups). Herein, the adenine nucleotide-silver mesh surface complexes have displayed a promising biosensing capacity.


 Please wait while we load your content...
Please wait while we load your content...