A novel analytical approach for the determination of parathion methyl in water: quadrupole isotope dilution mass spectrometry-dispersive liquid–liquid microextraction using multivariate optimization
Abstract
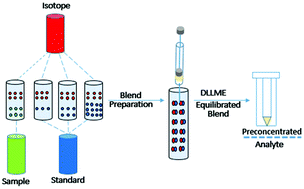
Dispersive liquid–liquid microextraction was coupled with quadruple isotope dilution mass spectrometry for the sensitive and accurate determination of parathion methyl in water. The two methods were complementary to each other, with DLLME preconcentrating the analyte for trace determination, and ID4MS maintaining the integrity of the method's accuracy and precision. An experimental design was used to optimize the extraction process. The results from the design were evaluated with the analysis of variance to determine the statistical significance of the main factors of extraction and interaction effects of these factors. A three-point calibration blend and sample blend were prepared gravimetrically by spiking with isotopically labelled parathion methyl. All four blends were left to equilibrate for three hours, after which they were preconcentrated under the optimum extraction conditions. The percent recovery recorded by this method was 99.9%, and the percent relative standard deviation was 0.32%. These results validated the accuracy and precision of the combined method.


 Please wait while we load your content...
Please wait while we load your content...