Reaction-based fluorescent probe for the selective and sensitive detection of thiophenols with a large Stokes shift and its application in water samples†
Abstract
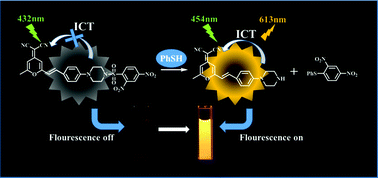
Although widely used in organic synthesis, pharmaceuticals and agrochemicals, thiophenol has brought about a series of ecological problems due to its high toxicity. Therefore, the development of efficient methods to discriminate thiophenols from aliphatic thiols is of great importance. In this work, a new reaction-based turn-on red fluorescence probe for the detection of thiophenols has been developed for the first time by employing dicyanomethylene-4H-pyran (DCM) as a fluorescence reporter and 2,4-dinitrobenzene-sulfonate (DNBS) as a recognition unit. The probe displayed a highly selective and sensitive (63 fold-fluorescence enhancement) response to thiophenols over aliphatic thiols. Additionally, the probe also exhibited a large Stokes shift (159 nm) and the detection limit reached as low as 8.3 nM. Moreover, this probe was also proved suitable for the quantification of thiophenol in real environmental water samples.


 Please wait while we load your content...
Please wait while we load your content...