Biosafety study and mechanism comparison on two types of silica with different nanostructures†
Abstract
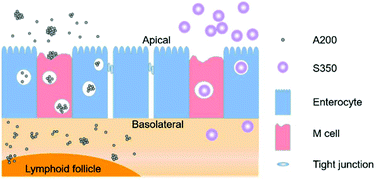
Silica is frequently used in oral drug delivery; however, its biosafety, particularly concerned with its nanostructure, has not been comprehensively studied yet. Here, the in vitro and in vivo biosafety of two types of silica (A200, nano-sized or micron-sized agglomerates; S350, micro-sized particles with nanopores) were compared and the possible reasons for the differences were explored. The results indicated that both A200 and S350 could inhibit the growth of Caco-2 cells by inducing apoptosis and changing the cell cycle progression. A200 showed a stronger influence than S350 in most of the in vitro experiments. In the in vivo study in KM mice, both A200 and S350 could change the blood constituents under the tested conditions; A200 also increased the levels of inflammatory factors in plasma and the numbers of CD4+ lymphocyte subsets. No obvious organic damage was observed in either the A200-treated or S350-treated groups. The transport study showed that neither A200 nor S350 were readily transported across the intestinal epithelial barrier in vitro and in vivo, but A200 could transport across the lymphatic-associated epithelium and accumulate in the Peyer's Patches, which might explain the A200-induced immune response. The increased transport of A200 might relate to its particle size, dispersion state and specific surface area. In conclusion, these results demonstrated that A200 and S350 exhibited diverse biosafety aspects, which correlated with their different nanostructures. We believe this study will provide some scientific information about the biosafety of A200 and S350 for their applications in oral drug delivery systems.


 Please wait while we load your content...
Please wait while we load your content...