Application research on PPARα-transgenic mice in preclinical safety evaluation of gemfibrozil
Abstract
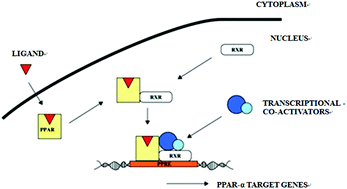
To explore the feasibility of peroxisome proliferator-activated receptor (PPAR)α transgenic mice applying in preclinical safety evaluation for peroxisome proliferators (PPs). Both PPARα transgenic mice and C57BL/6J mice were assigned as treated groups (PT and CT groups) and control groups (PC and CC groups). Gemfibrozil was administered into treated groups for 4 weeks. Body weight, blood biochemistry, enzyme activity and histological examinations were performed at scheduled time. The results showed that significant hypolipidaemic effects were induced in the treated groups after gemfibrozil treatment whereas the changes of non-esterified fatty acid and high density lipoproteincholesterol were different between the two treated groups. All the enzyme activities examined increased significantly in PT and CT groups except catalase which displayed no obvious change in the PT group. Pathology results showed a significant increase of the liver weight and the liver weight ratio in the CT group while no obvious changes were observed in the PT group. Hypertrophy of hepatocytes was discovered in CT and PT groups in histological examination, while the extent and incidence of hepatocyte hypertrophy in the CT group were higher than those in the PT group. The data suggest that PPARα transgenic mice could serve as a useful tool for preclinical safety assessment of PP drugs.

 Please wait while we load your content...
Please wait while we load your content...