Novel 4,8-benzobisthiazole copolymers and their field-effect transistor and photovoltaic applications†
Abstract
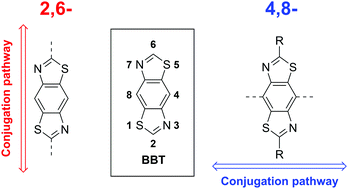
A series of copolymers containing the benzo[1,2-d:4,5-d′]bis(thiazole) (BBT) unit has been designed and synthesised with bisthienyl-diketopyrrolopyrrole (DPP), dithienopyrrole (DTP), benzothiadiazole (BT), benzodithiophene (BDT) or 4,4′-dialkoxybithiazole (BTz) comonomers. The resulting polymers possess a conjugation pathway that is orthogonal to the more usual substitution pathway through the 2,6-positions of the BBT unit, facilitating intramolecular non-covalent interactions between strategically placed heteroatoms of neighbouring monomer units. Such interactions enable a control over the degree of planarity through altering their number and strength, in turn allowing for tuning of the band gap. The resulting 4,8-BBT materials gave enhanced mobility in p-type organic field-effect transistors of up to 2.16 × 10−2 cm2 V−1 s−1 for pDPP2ThBBT and good solar cell performance of up to 4.45% power conversion efficiency for pBT2ThBBT.


 Please wait while we load your content...
Please wait while we load your content...