Valence band modification of Cr2O3 by Ni-doping: creating a high figure of merit p-type TCO†
Abstract
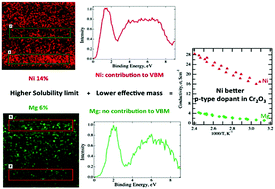
p-Type transparent conductors and semiconductors still suffer from remarkably low performance compared to their more widespread n-type counterparts, despite extensive investigation into their development. In this contribution, we present a comparative study on the defect chemistry of potential p-type transparent conducting oxides Mg-doped and Ni-doped Cr2O3. Conductivities as high as 28 S cm−1 were achieved by Ni-doping. By benchmarking crystallography and spectroscopy characterization against density functional theory calculations, we show that the incorporation of Ni into Cr2O3 contributes to the composition of the valence band, making the formed holes more delocalized, while Mg states do not interact with the valence band in Mg-doped Cr2O3. Furthermore, it is experimentally proven that Ni has a higher solubility in Cr2O3 than Mg, at least in the highly non-thermodynamic deposition conditions used for these experiments, which directly translates into a higher acceptor concentration. The combination of these two effects means that Ni is a more effective acceptor in Cr2O3 than Mg and explains the improved conductivity observed for the former.


 Please wait while we load your content...
Please wait while we load your content...