Solution-processable naphthalene and phenyl substituted carbazole core based hole transporting materials for efficient organic light-emitting diodes†
Abstract
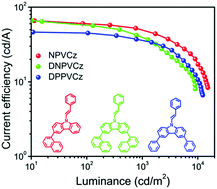
Solution-processable molecular hole transporting materials (HTMs) are extremely crucial in order to realize low cost, high throughput, and roll-to-roll fabrication of large area organic light emitting diodes for display and lighting applications. In this report, a series of naphthalene and phenyl substituted carbazole core based HTMs, 3-(1-naphthyl)-9-(2-phenylvinyl)carbazole (NPVCz), 3,6-di-(1-naphthyl)-9-phenylvinylcarbazole (DNPVCz), and 3,6-diphenyl-9-(2-phenylvinyl)carbazole (DPPVCz) are successfully synthesized and characterized. The synthesized HTMs possess excellent solubility in common organic solvents. By using a fluorescent tris(8-hydroxyquinolinato)aluminium emitter, we demonstrate an enhancement of 135%, from 1.7 to 4.5 cd A−1, in the current efficiency of an organic light emitting diode (OLED) by replacing the conventional HTM, N,N′-di(1-naphthyl)-N,N′-diphenyl-(1,1′-biphenyl)-4,4′-diamine (NPB), with the NPVCz counterpart. Moreover, the current efficiency of a conventional tris[2-phenylpyridinato-C2,N]iridium(III) based phosphorescent green OLED device increases from 46.4 to 66.2 cd A−1 by substituting the NPB with NPVCz. These findings suggest that this type of solution-processable molecular HTM will be a promising contender for high efficiency OLED devices.


 Please wait while we load your content...
Please wait while we load your content...