Molecular design of thermally activated delayed fluorescent emitters for blue-shifted emission by methoxy substitution
Abstract
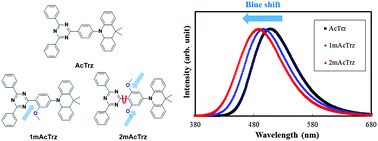
The molecular design method of thermally activated delayed fluorescent emitters for blue-shifted emission was developed by modifying a phenyl linker using a methoxy substituent. One methoxy or two methoxy groups were introduced into the phenyl linker connecting a diphenyltriazine acceptor and a dimethylacridine donor to manage the emission spectrum. Substitution of one methoxy group shifted the emission color to a short wavelength while preserving the external quantum efficiency of the pristine material without the methoxy substituent. A high external quantum efficiency of over 20% was obtained while blue-shifting the emission wavelength by 12 nm. However, the substitution of two methoxy groups decreased the quantum efficiency of the devices although the emission color was shifted to a short wavelength.


 Please wait while we load your content...
Please wait while we load your content...