Tetraphenylethylene tetracylhydrazine macrocycle with capability for discrimination of n-propanol from i-propanol and highly sensitive/selective detection of nitrobenzene†
Abstract
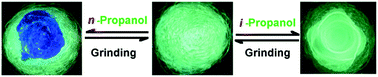
A novel tetraphenylethylene tetracylhydrazine macrocycle with an aggregation-induced emission effect was synthesized. Due to cyclization at the ortho position of the phenyl ring, the two TPE units that composed the macrocycle were almost folded and offered a deep V-like cavity. By grinding, the macrocycle powder could be made to emit yellow-green rather than blue-green fluorescence. Interestingly, the ground powder that emitted yellow-green fluorescence could be caused to emit blue light by methanol, ethanol or n-propanol while other conventional solvents including i-propanol did not cause this solvatochromism. Therefore, the macrocycle could discriminate between n-propanol and i-propanol. Moreover, the fluorescence of a suspension of the macrocycle in a mixed H2O/THF solvent was more easily quenched by nitrobenzene than by other nitroaromatic compounds, probably due to the smaller size of nitrobenzene instead of its greater electron-deficiency. The detection limit for the measurement of nitrobenzene was as low as 3.99 μg L−1, which has potential for usage in the analysis of nitrobenzene in potable water sources. Moreover, a solid film of the macrocycle showed a sensitive response to nitrobenzene vapour at a concentration as low as 1.01 femtograms per mL of air.


 Please wait while we load your content...
Please wait while we load your content...