Suppression of electrical conductivity and switching of conduction mechanisms in ‘stoichiometric’ (Na0.5Bi0.5TiO3)1−x(BiAlO3)x (0 ≤ x ≤ 0.08) solid solutions
Abstract
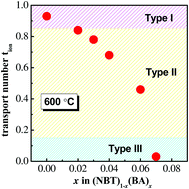
(Na0.5Bi0.5TiO3)1−x(BiAlO3)x (0 ≤ x ≤ 0.08) solid solutions were prepared by a solid state reaction and their electrical properties were established by ac impedance spectroscopy and electromotive force transport number measurements. Incorporation of BiAlO3 (BA) decreases the electrical conductivity of Na0.5Bi0.5TiO3 (NBT) and sequentially changes the conduction mechanism with increasing x from predominant oxide-ion conduction to mixed ionic–electronic conduction and finally to predominant electronic conduction. The suppressed oxide-ion conduction by BA incorporation significantly reduces the dielectric loss at elevated temperatures and produces excellent high-temperature dielectric materials for high BA contents. The possible reasons for the suppressed oxide-ion conduction in the NBT–BA solid solutions have been discussed and we propose that the local structure, especially trapping of oxygen vacancies by Al3+ on the B-site, plays a key role in oxide-ion conduction in these apparently ‘stoichiometric’ NBT-based solid-solution perovskite materials.


 Please wait while we load your content...
Please wait while we load your content...