The contributions of molecular vibrations and higher triplet levels to the intersystem crossing mechanism in metal-free organic emitters†
Abstract
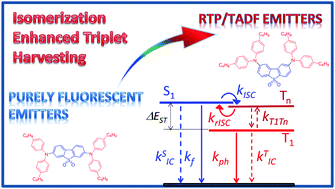
Dual luminescence, i.e. intense, simultaneous, room temperature phosphorescence (RTP) and thermally activated delayed fluorescence (TADF) is observed in a series of donor–acceptor–donor (D–A–D) molecules. This dual luminescence is stronger in the “angular” isomers, compared to their “linear” regioisomers, which is consistent with an enhanced intersystem crossing (ISC) in the former. Herein, we demonstrate that the small energy gap between the triplet levels, T1–Tn, below the lowest singlet state, S1, in the “angular” regioisomers, enhances the coupling between S1 and T1 states and favors ISC and reverse ISC (rISC). This is consistent with a spin–vibronic mechanism. In the absence of this “triplet ladder”, due to the larger energy difference between T1 and Tn in the “linear” regioisomers, the ISC and rISC are not efficient. Remarkably, the enhancement of the ISC rate in the “angular” regioisomers is accompanied by an increase of the rate of internal conversion (IC). These results highlight the contributions of higher triplet excited states and molecular vibronic coupling to the harvest of triplet states in organic compounds, and cast the TADF and RTP mechanisms into a common conceptual framework.


 Please wait while we load your content...
Please wait while we load your content...