Towards enhancing spin states in doped arylamine compounds through extended planarity of the spin coupling moieties†
Abstract
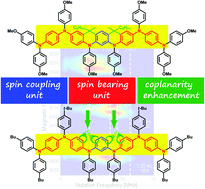
Arylamine moieties oxidized to radical cations are promising spin bearers for organic high-spin compounds. However, successive oxidations of next-neighbour sites as well as the resulting ferromagnetic exchange interactions depend strongly on the charge and spin distributions over the molecule. We report here that the use of rigid segments composed of m-phenylene spin couplers linked to arylamine spin bearers in a co-planar way facilitates successive oxidations, enhances spin exchange interactions and doubles the observed spin state (S = 2) in a polymer (PQA) when compared to the chemically equivalent (but locally free rotating) PA2 polymer (S = 1). Such a quintet spin state is observed for the first time for a linear polyarylamine with an m-phenylene coupler. DFT calculations of the dimer QA reproduce the experimental J value and indicate that oxidation of these co-planar compounds leads indeed to well localized radical cations, a fact of crucial importance for the preparation of arylamine-type high-spin materials.


 Please wait while we load your content...
Please wait while we load your content...