Ba6Li2CdSn4S16: lithium substitution simultaneously enhances band gap and SHG intensity†
Abstract
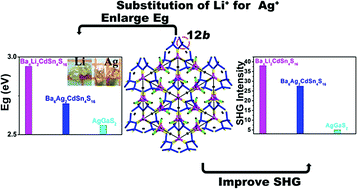
Herein, a new noncentrosymmetric (NCS) compound Ba6Li2CdSn4S16 (1) was discovered. As compared to its isostructural Ba6Ag2CdSn4S16 (2), compound 1 with Li substitution exhibited enlarged band gap (1: 3.02 eV vs.2: 2.70 eV) as well as enhanced second harmonic generation intensity (SHG, 1: 7.6 vs.2: 5.3 × AgGaS2 at 2.05 μm laser radiation). This is rare because the band gap of an NLO compound is usually inversely related to the SHG intensity. Both structures were chararcterized by single crystal X-ray diffraction techniques. The three-dimensional (3D) frameworks are constructed by corner-sharing SnS4 and (Li/Cd)S4 or (Ag/Cd)S4 tetrahedra with Ba2+ as counter cations. Density functional theory calculations (DFT) have confirmed that Li+ plays a significant role in the widening of the band gap and enhancement of the SHG response due to its strong ionicity. Because Li has small electronegativity and strong ionic bond nature, in comparison with that of 2, the top of the VB in 1 is driven down to lower energy regions to widen Eg, and the non-bonding ingredients of S-3p states are increased in 1 to produce stronger SHG intensity. The calculated d23 for 1 and 2 are 27.81 and 17.30 pm V−1 respectively, that agree with the experimental observations.


 Please wait while we load your content...
Please wait while we load your content...