Mixed ionic–electronic conduction in K1/2Bi1/2TiO3
Abstract
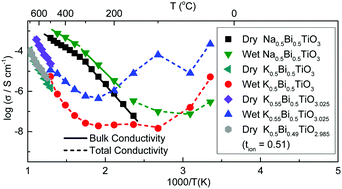
Recently, it has been reported that the Pb-free piezoelectric perovskite Na1/2Bi1/2TiO3 (NBT) can be compositionally tuned by close control of the A-site starting stoichiometry to exhibit high levels of oxide-ion conduction. The related K1/2Bi1/2TiO3 (KBT) perovskite has also drawn considerable interest as a promising Pb-free piezoelectric material; however, its conduction properties have been less extensively investigated. Here we report on the influence of the K/Bi ratio in the starting composition on the electrical properties using a combination of impedance spectroscopy and ion-transport property measurements. KBT ceramics exhibit mixed ionic–electronic (oxide-ion) conduction with tion ∼ 0.5 at 600–800 °C and although variations in the A-site starting stoichiometry can create a ∼1 order of magnitude difference in the bulk conductivity at >500 °C, the conductivity is low (ca. 0.1 to 1 mS cm−1 at 700 °C) and the activation energy for bulk conduction remains in the range ∼1.2 to 1.5 eV. The high temperature electrical transport properties of KBT are therefore much less sensitive to the starting A-site stoichiometry as compared to NBT. However, KBT ceramics exhibit non-negligible proton conduction at lower temperatures (<300 °C). For K/Bi ≥ 1 the total conductivity of KBT ceramics at room temperature can be as high as ∼0.1 mS cm−1 under wet atmospheric conditions. This study demonstrates ionic conduction to be a common feature in A1/2Bi1/2TiO3 perovskites, where A = Na, K.
- This article is part of the themed collection: 2017 Journal of Materials Chemistry C HOT Papers


 Please wait while we load your content...
Please wait while we load your content...