Circularly polarized luminescence of chiral 1,8-naphthalimide-based pyrene fluorophore induced via supramolecular self-assembly†
Abstract
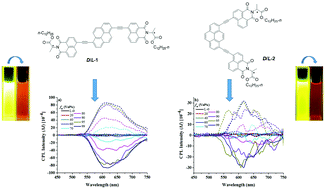
Herein, two pairs of chiral 1,8-naphthalimide-based enantiomers incorporating D/L-alanine and pyrene fluorophore moieties were designed and synthesized. The fluorescence emission gradually changes from bright-yellow to red when the fraction of the poor solvent methanol increases from 0 to 99 vol%. No obvious circular dichroism (CD) and circularly polarized luminescence (CPL) signals could be observed in the CHCl3 solution. Interestingly, D/L-1 can exhibit a stronger red-colored CPL response signal as compared to D/L-2 at fm = 99% in the aggregate state; this is due to the formation of regular and orderly self-assembled nanonetworks in the aggregate state via intermolecular π–π interactions. Moreover, the optical anisotropy factor (glum) could reach a value as high as 0.013.


 Please wait while we load your content...
Please wait while we load your content...