Mechanochromism and electroluminescence in positional isomers of tetraphenylethylene substituted phenanthroimidazoles†
Abstract
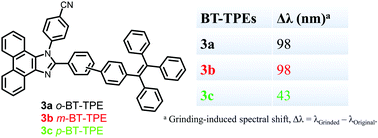
The study of aggregation-induced emission (AIE) luminogens has gained momentum due to their remarkable luminogenic properties and applications in mechano-sensors and organic light-emitting diodes (OLEDs). In this article we have studied three positional isomers (ortho, meta, and para) of phenanthroimidazoles 3a–3c and explored their AIE, mechanochromic and electroluminescence behavior. The phenanthroimidazoles 3a–3c were synthesized by the Suzuki cross-coupling reaction of (2-bromo/3-bromo/4-bromo)phenathroimidazoles 2a–2c with 4-(1,2,2-triphenylvinyl)phenylboronic acid pinacol ester in good yields. The phenanthroimidazoles 3a–3c exhibit strong AIE. The mechanochromic study reveals reversible mechanochromism with good color contrast between blue and green colors. The ortho (3a) and meta (3b) isomers exhibit a grinding induced spectral shift of 98 nm while the para-isomer (3c) exhibits a spectral shift of 43 nm. Moreover, 3a–3c were explored as non-doped blue emitters in efficient organic light-emitting diodes. Among the three emitters, 3c provided a high quantum efficiency of 4.0% in a non-doped blue device.


 Please wait while we load your content...
Please wait while we load your content...