Red-emitting Au7 nanoclusters with fluorescence sensitivity to Fe2+ ions
Abstract
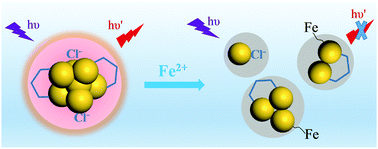
Luminescent gold nanoclusters have recently emerged as an important class of sensing and imaging materials in optical applications. Here we report a red-emitting gold nanocluster with a precise molecular formula of Au7(DHLA)2Cl2, synthesized via the size-focusing strategy using a bidentate ligand of dihydrolipoic acid (DHLA). This Au nanocluster is water-soluble and emits fluorescence at 683 nm with a quantum yield of 3.6% in water. The intense fluorescence of Au7(DHLA)2Cl2 originates from the aggregation of Au(I)–DHLA/Cl motifs on the Au(0) core in a rigid core/shell-like structure. Moreover, we find that trace levels of Fe2+ ions can quench selectively and sensitively the fluorescence of the Au7(DHLA)2Cl2 nanocluster, making it a potential fluorescent sensor for Fe2+ with a limit of detection of 3.8 μM (0.2 ppm). A dissociation-induced fluorescence quenching mechanism is also proposed to describe the fluorescence response of the nanocluster to Fe2+ ions.


 Please wait while we load your content...
Please wait while we load your content...