New 3,3′-(ethane-1,2-diylidene)bis(indolin-2-one) (EBI)-based small molecule semiconductors for organic solar cells
Abstract
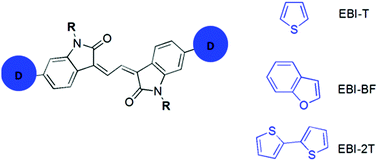
A series of new donor–acceptor–donor (D–A–D) small-molecule compounds, with 3,3′-(ethane-1,2-diylidene)bis(indolin-2-one) (EBI) as an electron acceptor building block coupled with various electron donor end-capping moieties (thiophene, bithiophene and benzofuran), were synthesized and characterized. When the fused-ring benzofuran is combined to EBI (EBI-BF), the molecules displayed a perfectly planar conformation and afforded the best charge transport properties among these EBI compounds with a hole mobility of up to 0.02 cm2 V−1 s−1. All EBI-based small molecules were used as donor material along with a PC61BM acceptor for the fabrication of solution-processed bulk-heterojunction (BHJ) solar cells. The best performing photovoltaic devices are based on the EBI derivative using the bithiophene end-capping moiety (EBI-2T) with a maximum power conversion efficiency (PCE) of 1.92%, owing to the broad absorption spectra of EBI-2T and the appropriate morphology of the BHJ. With the aim of establishing a correlation between the molecular structure and the thin film morphology, differential scanning calorimetry, atomic force microscopy and X-ray diffraction analysis were performed on neat and blend films of each material.


 Please wait while we load your content...
Please wait while we load your content...