A comparative study of carbazole-based thermally activated delayed fluorescence emitters with different steric hindrance†
Abstract
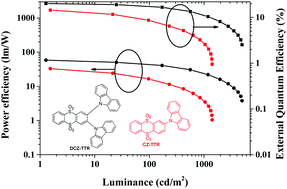
Thermally activated delayed fluorescence (TADF) emitters based on carbazole groups have been reported to realize different efficiencies in devices. Herein, we report two carbazole-based TADF emitters, namely 2-(9H-carbazol-9-yl)thianthrene 5,5,10,10-tetraoxide (CZ-TTR), which has one free-rotation carbazole, and 2,3-di(9H-carbazol-9-yl)thianthrene 5,5,10,10-tetraoxide (DCZ-TTR), which has two mutually restricted carbazole groups, and investigated the influence of steric hindrance on their properties. Both compounds employed the same donor and acceptor segments and connecting mode. However, due to steric hindrance between the two carbazole segments, DCZ-TTR exhibited a smaller singlet–triplet splitting of 0.03 eV compared with that of CZ-TTR (0.10 eV). The device containing DCZ-TTR showed significantly higher efficiencies (20.1% for external quantum efficiency (EQE), 58.5 lm W−1 for power efficiency (PE), and 59.6 cd A−1 for current efficiency (CE)) than those of the CZ-TTR-based device (EQE = 14.4%; PE = 32.9 lm W−1; CE = 32.5 cd A−1). These results clearly proved the necessity of introducing suitable steric hindrance when designing highly efficient TADF emitters based on carbazole groups.


 Please wait while we load your content...
Please wait while we load your content...