A general and facile approach to disperse hydrophobic nanocrystals in water with enhanced long-term stability†
Abstract
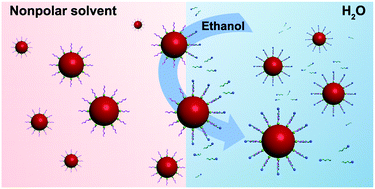
We report a general and fast approach that can transfer any hydrophobic nanocrystals into water within several seconds without vigorous stirring or ultrasonication treatment at room temperature. The key factor is to find a suitable solvent that can be soluble in both organic solvent and water using the classical “like dissolves like” rule. Hydrophobic nanocrystals covered with oleic acid, oleylamine, and TOPO have been successfully transferred into water. The second ligand could be any amphiphilic ligands, such as sodium oleate, decanoate, dopamine, CTAB, and so on. The surface charge could be tuned to negatively or positively charged by using different ligands. After phase transfer, the original properties, including the plasmonic, magnetic, catalytic, and fluorescence properties, of the nanocrystals are well retained.


 Please wait while we load your content...
Please wait while we load your content...