Anomalous spontaneous-reduction of Mn7+/Mn4+ to Mn2+ and luminescence properties in Zn2GeO4:Mn†
Abstract
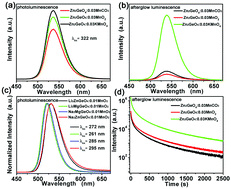
The reduction-atmosphere of CO, H2/N2 or H2 is commonly used for the synthesis of divalent manganese ion (Mn2+) activated luminescent materials. Herein, by using KMnO4 or MnO2 as the manganese source, the spontaneous-reduction of Mn7+ or Mn4+ to Mn2+ in air with intense Mn2+-emission peaking at 536 nm has been first demonstrated in Zn2GeO4 synthesized by a high temperature solid state reaction method. The effects of synthetic conditions including Mn sources (MnCO3, MnO2 or KMnO4) and doping concentration on the crystal structure, spontaneous-reduction behaviors, photoluminescence and long-persistent luminescence properties have been investigated and discussed in detail. Based on the static and dynamic spectral measurements, electron paramagnetic resonance (EPR), XPS and crystal structure characterization, a charge compensation mechanism has been proposed to interpret the spontaneous-reduction phenomenon of Mn4+(Mn7+) to Mn2+ in Zn2GeO4, which is extended to some other germanates A2BGeO4 (A = Li, Na; B = Zn, Mg) as well. These findings would be beneficial not only for developing a low-cost and safe strategy to produce Mn2+ activated luminescent materials but also for providing new insights into improving the photoluminescence and long-persistent luminescence properties of Mn2+.


 Please wait while we load your content...
Please wait while we load your content...