Adamantane substitutions: a path to high-performing, soluble, versatile and sustainable organic semiconducting materials†
Abstract
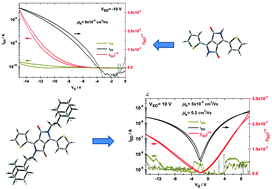
Novel ethyladamantyl solubilization side groups were found to induce π–π interactions between the conjugated cores through adamantyl–adamantyl stacking in soluble diketopyrrolopyrrole (DPP) derivatives. The closeness of the DPP cores amplifies charge transfer in the material, as far as the π–π interaction is a dominant charge-hopping pathway. As a result, tenfold enhancement of hole mobilities exceeding those obtained for insoluble derivatives was reached. Moreover, due to high crystallinity and co-planarity of the conjugated cores, electron transfer was preserved with a mobility of 0.2 cm2 V−1 s−1 for dithiophene-DPP. At the same time, the material remained soluble, which is a significant advantage for purification and processing. This approach can be universally applied for many types of semiconducting organic materials containing the imide motif, where solubilization is achieved by side-group substitution.


 Please wait while we load your content...
Please wait while we load your content...