Molecularly imprinted polymer based extended-gate field-effect transistor chemosensors for phenylalanine enantioselective sensing†
Abstract
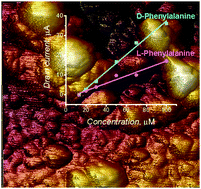
Chemosensing systems were devised for the enantioselective determination of D- and L-phenylalanine (D- and L-Phe). As recognition units of these systems, molecularly imprinted polymers (MIPs) were designed, guided by DFT calculations, and then synthesized. For the preparation of these MIPs, carboxy derivatized bis(bithiophene) was used as the functional monomer. Both templated and template-extracted MIP films as well as non-imprinted polymer (NIP) films were characterized by IR spectroscopy to prove Phe templation, and then extraction. Extended-gate field-effect transistors (EG-FETs) served as transducers. The EG-FET gates were coated with D- or (L-Phe)-templated MIP films, by electropolymerization, to result in complete chemosensors. These chemosensors rapidly and selectively responded to D- and L-Phe enantiomer analytes. They readily discriminated between a homologous series of analytes differing by a single atom as well as pairs of enantiomers differing in their three-dimensional structures. Linear dynamic concentration ranges for D- and L-Phe extended from 13 to 100 μM. For both Phe enantiomers, the limit of detection was 13 μM. The enantioselectivity factor was ∼2.3 for both chemosensors.

 Please wait while we load your content...
Please wait while we load your content...