Enzyme-instructed self-assembly leads to the activation of optical properties for selective fluorescence detection and photodynamic ablation of cancer cells†
Abstract
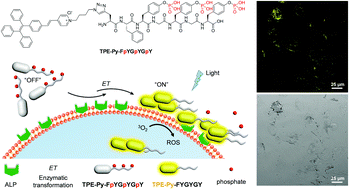
Both fluorescence and photoactivity activatable probes are particularly valuable for cancer theranostics as they allow for sensitive fluorescence diagnosis and on-demand photodynamic therapy (PDT) against targeted cancer cells at the same time, which undoubtedly promote the diagnostic accuracy and reduce the side effects on normal tissues/cells. Here, we show that enzyme-instructed self-assembly (EISA) is an ideal strategy to develop a both fluorescence and reactive oxygen species (ROS) generation capability activatable probe with aggregation-induced emission (AIE) signature. As a proof-of-concept, we design and synthesize a precursor TPE-Py-FpYGpYGpY that consists of an AIE luminogen (TPE-Py) and a short peptide with three tyrosine phosphates (pY), which permits selective fluorescence visualization and PDT of alkaline phosphatase (ALP)-overexpressed cancer cells. TPE-Py-FpYGpYGpY has good aqueous solubility thanks to the hydrophilic phosphotyrosine residues and hence leads to weak fluorescence and negligible ROS generation ability. After ALP enzymatic dephosphorylation of the precursors, however, self-assembly of ALP-catalysed products occurs and the resultant nanostructures are activated to be highly emissive and efficiently produce ROS. Cellular studies reveal that TPE-Py-FpYGpYGpY is capable of differentiating cancer cells and normal cells, specifically pinpointing and suppressing ALP-overexpressed cancer cells. This study may inspire new insights into the design of advanced activatable molecular probes.
- This article is part of the themed collection: Cancer Diagnostics


 Please wait while we load your content...
Please wait while we load your content...