Stable, polyvalent aptamer-conjugated near-infrared fluorescent nanocomposite for high-performance cancer cell-targeted imaging and therapy†
Abstract
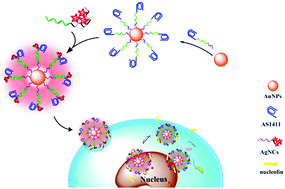
Early diagnosis and targeted therapy are two important ways to improve cancer treatment survival. In this work, via a simple DNA hybridization reaction, DNA-templated fluorescent silver nanoclusters (AgNCs) were successfully assembled around a DNA-modified gold nanoparticle (AuNP) to construct a novel nanocomposite with the functions of cancer cell-specific imaging and targeted therapy. The as-prepared AuNP@(AS1411–AgNCs)n nanocomposite shows strong near-infrared fluorescence emission and improved biostability, and carries a high density of AS1411—the first anticancer aptamer targeting nucleolin protein, which is over-expressed not only on the surface but also in the cytoplasm of cancer cells. The synergy of multivalent AS1411–nucleolin binding promotes accumulation of the nanocomposite towards cancer cells and subsequent internalization in them. This results in highly specific cancer cell-targeted imaging and selective killing in a low AuNP@(AS1411–AgNCs)n concentration range. The prepared nanocomposite also shows great potential as a carrier of doxorubicin to further promote the selective killing of cancer cells.


 Please wait while we load your content...
Please wait while we load your content...