Formation of toroids by self-assembly of an α–α corner mimetic: supramolecular cyclization†
Abstract
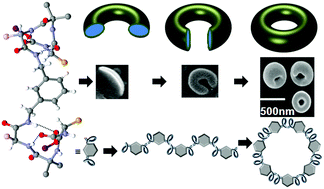
An α–α corner mimetic self-assembles to form a rod-like supramolecular structure which bends and closes end-to-end like a cyclization reaction to form uniform toroids. Each peptide fragment containing L-leucine, α-aminoisobutyric acid (Aib) and L-tyrosine forms rigid 310 helical structures stabilized by multiple intramolecular N–H⋯O hydrogen bonds. Two 310 helices are connected by the spacer 3-aminomethyl-benzylamine and maintain an angular distance of 120° and therefore mimic the α–α corner motif of a protein super secondary structure. The individual α–α corner subunits are themselves regularly interlinked through multiple water mediated intermolecular hydrogen-bonding interactions to form the rod-like supramolecular structure and toroids. The formation of the supramolecular structure has been proven with X-ray crystallography and other spectroscopic techniques. The cyclization of the supramolecular structure and toroid formation were studied by optical microscope, AFM and FE-SEM experiments. Despite other assignments such as exfoliation of graphene from graphite, the compound exhibits significant memory to finally produce the toroids.


 Please wait while we load your content...
Please wait while we load your content...