Gold–iron oxide dimers for magnetic hyperthermia: the key role of chloride ions in the synthesis to boost the heating efficiency†
Abstract
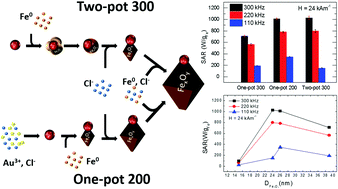
With the aim of producing Au–FexOy dimers with outstanding heating performances under magnetic hyperthermia conditions applicable to human patients, here we report two synthesis routes, a two-pot and a one-pot method. The addition of chloride ions and the absence of 1,2-hexadecanediol (HDDOL), a commonly used chemical in this synthesis, are the key factors that enable us to produce dimers at low temperature with crystalline iron oxide domains in the size range between 18–39 nm that is ideal for magnetic hyperthermia. In the case of two-pot synthesis, in which no chloride ions are initially present in the reaction pot, dimers are obtained only at 300 °C. In order to lower the reaction temperature to 200 °C and to tune the size of the iron oxide domain, the addition of chloride ions becomes the crucial parameter. In the one-pot method, the presence of chloride ions from the start of the synthesis (as counter ions of the gold salt precursor) enables a prompt formation of dimers directly at 200 °C. In this case, the reaction time is the main parameter used to tune the iron oxide size. A record value of specific absorption rates (SARs) up to 1300 W gFe−1 at 330 kHz and 24 kA m−1 was measured for dimers with an iron oxide domain of 24 nm in size.

 Please wait while we load your content...
Please wait while we load your content...