Enantioseparation performance of CNTs as chiral selectors for the separation of ibuprofen isomers: a dispersion corrected DFT study
Abstract
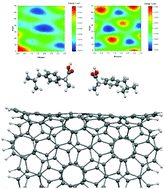
The enantioseparation of chiral drugs has been of great interest in the modern pharmaceutical industry since the majority of bioorganic compounds are chiral. In this work, we have investigated the ability of pristine and defected (10, 5) chiral carbon nanotubes (CNTs) in enantioseparation of chiral R-/S-ibuprofen isomers. The interactions between the two enantiomers of ibuprofen and the outer surface and inner side wall of the chiral CNTs have been evaluated. We utilized dispersion-corrected density functional theory (DFT) calculations within the framework of the GGA-PBE scheme for the systems under study. The results indicated that the inner side walls of the defected (10, 5) CNTs exhibited the highest energy difference (ΔU0) between the pairs of considered enantiomers with the energy difference of about 1.4 kcal mol−1, indicating that these nanotubes are a promising candidate in enantioseparation processes. The effect of solvation has also been considered in the calculations and it was found that changing the dielectric properties of the medium cannot affect the overall interactions between the drug and CNT. The electronic properties of the considered systems did not change upon the interaction between the incorporated molecules and the type of interaction was found to be dispersion-governed physisorption.


 Please wait while we load your content...
Please wait while we load your content...